Clinical Trials FAQ
General Information about Clinical Trials
Clinical Trials FAQ
General Information about Clinical Trials
What is a clinical trials?
What is the difference between an observational and interventional clinical trial?
Who runs clinical trials?
Clinical trials are run by clinical researchers, typically at universities or research hospitals. Clinical researchers will have an advanced degree in their field, like a MD and/or PhD. This means that they have spent many years learning about their field of study and conducting research which makes them experts in their field. They will typically design research studies and clinical trials based on previous research and data, usually in collaboration with other experts.
Clinical trials are often sponsored by different groups, including government agencies like the NIH, pharmaceutical or biotech companies, academic institutions, and nonprofit or philanthropic organizations.
What are the different phases of a clinical trial?
There are 4 phases of a clinical trial, each designed to answer specific questions about a new treatment’s safety and effectiveness. The goal of each phase of a clinical trial is distinct and builds upon the previous phase.
Phase 1: A Phase 1 clinical trial primarily focuses on evaluating the safety of a new treatment, identifying potential side effects, and determining the appropriate dosage in a small group of participants, after undergoing extensive tests in the laboratory. Here, researchers start with low doses and gradually increase them while carefully monitoring participants for adverse reactions. Although Phase 1 trials are not primarily designed to assess effectiveness, any preliminary signs of benefit are noted.
This phase lays the foundation for subsequent trials by establishing the safest and most effective dose to use moving forward.
Phase 2: A Phase 2 clinical trial builds on the safety data from Phase 1 by evaluating whether the treatment is effective, while continuing to monitor for side effects. This phase typically involves a larger group of participants who have the condition the treatment is meant to target.
Researchers use the dose determined in Phase 1 to assess how well the treatment works. Although not yet definitive, Phase 2 trials help determine whether the treatment shows enough promise to move forward into larger, confirmatory studies.
Phase 3: A Phase 3 clinical trial builds on the results from earlier phases by confirming the treatment’s effectiveness in a much larger group of patients. It compares the new treatment to the current standard of care to determine which works better, while continuing to monitor for side effects.
This phase provides the definitive evidence needed to assess the treatment’s overall benefit-risk profile and is often the final step before seeking Federal Food and Drug Administration (FDA) regulatory approval.
Phase 4: This phase takes place after a treatment is approved and widely available, focusing on long-term safety, effectiveness, and outcomes in diverse, real-world populations, sometimes involving thousands of participants. It involves ongoing monitoring which helps identify rare or delayed side effects and ensures continued assessment of the treatment’s overall benefit and risk.
For PTCL patients, clinical trials will typically be in Phase 1 or 2. Phase 3 trials are limited due to the fact that PTCL is a rare condition, and it is difficult to find enough patients to participate. Medications for PTCL have historically been approved from Phase 2 trials.
How safe are clinical trials?
All clinical trials involve some degree of risk for participants, which is an important factor to consider before enrolling. The amount of risk varies from trial to trial. Some of these risks can include mild to more severe side effects of the medication. That being said, the FDA approves all interventional trials before they become activated, and prioritizes the safety of the patients over all else. Researchers running the clinical trials are also there to ensure the safety of all participants during the trial.
For more information, visit this NIH web page.
What is a placebo treatment? Why are they important?
A placebo treatment is a control treatment where the participant is not given the new experimental treatment. Instead, the participant is given the current standard of care treatment. In almost all clinical trials, participants are randomly assigned by a computer to receive either the new experimental treatment or the current standard of care. This is called a “blind” trial, because if participants or researchers choose which treatment each participant gets, the results will likely become biased and less credible. Often, clinical trials have a “double blind” design, where both participants and researchers do not know who is receiving which treatment. The information about who received which treatment is often revealed at the very end of the trial, when researchers begin to analyze their results, in order to prevent bias and ensure that the results of the trial are credible.
Although it may be disappointing to not know if you are actually receiving the new treatment or not–being a participant in the trial, even if you are just receiving a placebo treatment–is incredibly valuable to furthering knowledge about T-cell lymphoma and improving outcomes for yourself and patients in the future.
Click here to view image.
What are the pros and cons of participating in a clinical trial?
Ultimately, it is your choice to decide whether or not to participate in a clinical trial. Here are some general questions to think about and discuss with your care team:
1) If I participate in a clinical trial, will my chances of improving my lymphoma be greater, less, or equal to my chances if I continue with my current treatment?
2) Am I comfortable with the possible health risks and side effects that may come with receiving a new drug? Does this risk outweigh the health risks and side effects of my current treatment?
3) Do I have the financial and personal support to participate in a clinical trial? Do I have a reliable way to get to and from the clinical trial center for several months or years?
4) Do I have support from someone to take care of me if I become too ill to take care of myself? Do I have personal support from family and friends, as well as support from a physician or care team?
Most of the time, these questions are difficult to answer confidently. It is okay to have questions and to take time to make your decision before participating in a clinical trial. Remember that it is alright to say yes and also alright to say no, as long as you feel comfortable with your informed decision.
How do I find a physician or clinic that can support me in the clinical trial process?
If your current physician doesn’t offer a clinical trial that fits your needs, they can help refer you to another doctor or center that may have more options available.
You can also check this list of PTCL Cancer Centers to see if there is a cancer center near you who can help you with receiving care
What is a clinical trials?
What is the difference between an observational and interventional clinical trial?
Who runs clinical trials?
Clinical trials are run by clinical researchers, typically at universities or research hospitals. Clinical researchers will have an advanced degree in their field, like a MD and/or PhD. This means that they have spent many years learning about their field of study and conducting research which makes them experts in their field. They will typically design research studies and clinical trials based on previous research and data, usually in collaboration with other experts.
Clinical trials are often sponsored by different groups, including government agencies like the NIH, pharmaceutical or biotech companies, academic institutions, and nonprofit or philanthropic organizations.
What are the different phases of a clinical trial?
There are 4 phases of a clinical trial, each designed to answer specific questions about a new treatment’s safety and effectiveness. The goal of each phase of a clinical trial is distinct and builds upon the previous phase.
Phase 1: A Phase 1 clinical trial primarily focuses on evaluating the safety of a new treatment, identifying potential side effects, and determining the appropriate dosage in a small group of participants, after undergoing extensive tests in the laboratory. Here, researchers start with low doses and gradually increase them while carefully monitoring participants for adverse reactions. Although Phase 1 trials are not primarily designed to assess effectiveness, any preliminary signs of benefit are noted.
This phase lays the foundation for subsequent trials by establishing the safest and most effective dose to use moving forward.
Phase 2: A Phase 2 clinical trial builds on the safety data from Phase 1 by evaluating whether the treatment is effective, while continuing to monitor for side effects. This phase typically involves a larger group of participants who have the condition the treatment is meant to target.
Researchers use the dose determined in Phase 1 to assess how well the treatment works. Although not yet definitive, Phase 2 trials help determine whether the treatment shows enough promise to move forward into larger, confirmatory studies.
Phase 3: A Phase 3 clinical trial builds on the results from earlier phases by confirming the treatment’s effectiveness in a much larger group of patients. It compares the new treatment to the current standard of care to determine which works better, while continuing to monitor for side effects.
This phase provides the definitive evidence needed to assess the treatment’s overall benefit-risk profile and is often the final step before seeking Federal Food and Drug Administration (FDA) regulatory approval.
Phase 4: This phase takes place after a treatment is approved and widely available, focusing on long-term safety, effectiveness, and outcomes in diverse, real-world populations, sometimes involving thousands of participants. It involves ongoing monitoring which helps identify rare or delayed side effects and ensures continued assessment of the treatment’s overall benefit and risk.
For PTCL patients, clinical trials will typically be in Phase 1 or 2. Phase 3 trials are limited due to the fact that PTCL is a rare condition, and it is difficult to find enough patients to participate. Medications for PTCL have historically been approved from Phase 2 trials.
How safe are clinical trials?
All clinical trials involve some degree of risk for participants, which is an important factor to consider before enrolling. The amount of risk varies from trial to trial. Some of these risks can include mild to more severe side effects of the medication. That being said, the FDA approves all interventional trials before they become activated, and prioritizes the safety of the patients over all else. Researchers running the clinical trials are also there to ensure the safety of all participants during the trial.
For more information, visit this NIH web page.
What is a placebo treatment? Why are they important?
A placebo treatment is a control treatment where the participant is not given the new experimental treatment. Instead, the participant is given the current standard of care treatment. In almost all clinical trials, participants are randomly assigned by a computer to receive either the new experimental treatment or the current standard of care. This is called a “blind” trial, because if participants or researchers choose which treatment each participant gets, the results will likely become biased and less credible. Often, clinical trials have a “double blind” design, where both participants and researchers do not know who is receiving which treatment. The information about who received which treatment is often revealed at the very end of the trial, when researchers begin to analyze their results, in order to prevent bias and ensure that the results of the trial are credible.
Although it may be disappointing to not know if you are actually receiving the new treatment or not–being a participant in the trial, even if you are just receiving a placebo treatment–is incredibly valuable to furthering knowledge about T-cell lymphoma and improving outcomes for yourself and patients in the future.
Click here to view image.
What are the pros and cons of participating in a clinical trial?
Ultimately, it is your choice to decide whether or not to participate in a clinical trial. Here are some general questions to think about and discuss with your care team:
1) If I participate in a clinical trial, will my chances of improving my lymphoma be greater, less, or equal to my chances if I continue with my current treatment?
2) Am I comfortable with the possible health risks and side effects that may come with receiving a new drug? Does this risk outweigh the health risks and side effects of my current treatment?
3) Do I have the financial and personal support to participate in a clinical trial? Do I have a reliable way to get to and from the clinical trial center for several months or years?
4) Do I have support from someone to take care of me if I become too ill to take care of myself? Do I have personal support from family and friends, as well as support from a physician or care team?
Most of the time, these questions are difficult to answer confidently. It is okay to have questions and to take time to make your decision before participating in a clinical trial. Remember that it is alright to say yes and also alright to say no, as long as you feel comfortable with your informed decision.
How do I find a physician or clinic that can support me in the clinical trial process?
If your current physician doesn’t offer a clinical trial that fits your needs, they can help refer you to another doctor or center that may have more options available.
You can also check this list of PTCL Cancer Centers to see if there is a cancer center near you who can help you with receiving care
What is a clinical trials?
What is the difference between an observational and interventional clinical trial?
Who runs clinical trials?
Clinical trials are run by clinical researchers, typically at universities or research hospitals. Clinical researchers will have an advanced degree in their field, like a MD and/or PhD. This means that they have spent many years learning about their field of study and conducting research which makes them experts in their field. They will typically design research studies and clinical trials based on previous research and data, usually in collaboration with other experts.
Clinical trials are often sponsored by different groups, including government agencies like the NIH, pharmaceutical or biotech companies, academic institutions, and nonprofit or philanthropic organizations.
What are the different phases of a clinical trial?
There are 4 phases of a clinical trial, each designed to answer specific questions about a new treatment’s safety and effectiveness. The goal of each phase of a clinical trial is distinct and builds upon the previous phase.
Phase 1: A Phase 1 clinical trial primarily focuses on evaluating the safety of a new treatment, identifying potential side effects, and determining the appropriate dosage in a small group of participants, after undergoing extensive tests in the laboratory. Here, researchers start with low doses and gradually increase them while carefully monitoring participants for adverse reactions. Although Phase 1 trials are not primarily designed to assess effectiveness, any preliminary signs of benefit are noted.
This phase lays the foundation for subsequent trials by establishing the safest and most effective dose to use moving forward.
Phase 2: A Phase 2 clinical trial builds on the safety data from Phase 1 by evaluating whether the treatment is effective, while continuing to monitor for side effects. This phase typically involves a larger group of participants who have the condition the treatment is meant to target.
Researchers use the dose determined in Phase 1 to assess how well the treatment works. Although not yet definitive, Phase 2 trials help determine whether the treatment shows enough promise to move forward into larger, confirmatory studies.
Phase 3: A Phase 3 clinical trial builds on the results from earlier phases by confirming the treatment’s effectiveness in a much larger group of patients. It compares the new treatment to the current standard of care to determine which works better, while continuing to monitor for side effects.
This phase provides the definitive evidence needed to assess the treatment’s overall benefit-risk profile and is often the final step before seeking Federal Food and Drug Administration (FDA) regulatory approval.
Phase 4: This phase takes place after a treatment is approved and widely available, focusing on long-term safety, effectiveness, and outcomes in diverse, real-world populations, sometimes involving thousands of participants. It involves ongoing monitoring which helps identify rare or delayed side effects and ensures continued assessment of the treatment’s overall benefit and risk.
For PTCL patients, clinical trials will typically be in Phase 1 or 2. Phase 3 trials are limited due to the fact that PTCL is a rare condition, and it is difficult to find enough patients to participate. Medications for PTCL have historically been approved from Phase 2 trials.
How safe are clinical trials?
All clinical trials involve some degree of risk for participants, which is an important factor to consider before enrolling. The amount of risk varies from trial to trial. Some of these risks can include mild to more severe side effects of the medication. That being said, the FDA approves all interventional trials before they become activated, and prioritizes the safety of the patients over all else. Researchers running the clinical trials are also there to ensure the safety of all participants during the trial.
For more information, visit this NIH web page.
What is a placebo treatment? Why are they important?
A placebo treatment is a control treatment where the participant is not given the new experimental treatment. Instead, the participant is given the current standard of care treatment. In almost all clinical trials, participants are randomly assigned by a computer to receive either the new experimental treatment or the current standard of care. This is called a “blind” trial, because if participants or researchers choose which treatment each participant gets, the results will likely become biased and less credible. Often, clinical trials have a “double blind” design, where both participants and researchers do not know who is receiving which treatment. The information about who received which treatment is often revealed at the very end of the trial, when researchers begin to analyze their results, in order to prevent bias and ensure that the results of the trial are credible.
Although it may be disappointing to not know if you are actually receiving the new treatment or not–being a participant in the trial, even if you are just receiving a placebo treatment–is incredibly valuable to furthering knowledge about T-cell lymphoma and improving outcomes for yourself and patients in the future.
Click here to view image.
What are the pros and cons of participating in a clinical trial?
Ultimately, it is your choice to decide whether or not to participate in a clinical trial. Here are some general questions to think about and discuss with your care team:
1) If I participate in a clinical trial, will my chances of improving my lymphoma be greater, less, or equal to my chances if I continue with my current treatment?
2) Am I comfortable with the possible health risks and side effects that may come with receiving a new drug? Does this risk outweigh the health risks and side effects of my current treatment?
3) Do I have the financial and personal support to participate in a clinical trial? Do I have a reliable way to get to and from the clinical trial center for several months or years?
4) Do I have support from someone to take care of me if I become too ill to take care of myself? Do I have personal support from family and friends, as well as support from a physician or care team?
Most of the time, these questions are difficult to answer confidently. It is okay to have questions and to take time to make your decision before participating in a clinical trial. Remember that it is alright to say yes and also alright to say no, as long as you feel comfortable with your informed decision.
How do I find a physician or clinic that can support me in the clinical trial process?
If your current physician doesn’t offer a clinical trial that fits your needs, they can help refer you to another doctor or center that may have more options available.
You can also check this list of PTCL Cancer Centers to see if there is a cancer center near you who can help you with receiving care
How Do I Apply For Clinical Trails?

How do I find clinical trials?
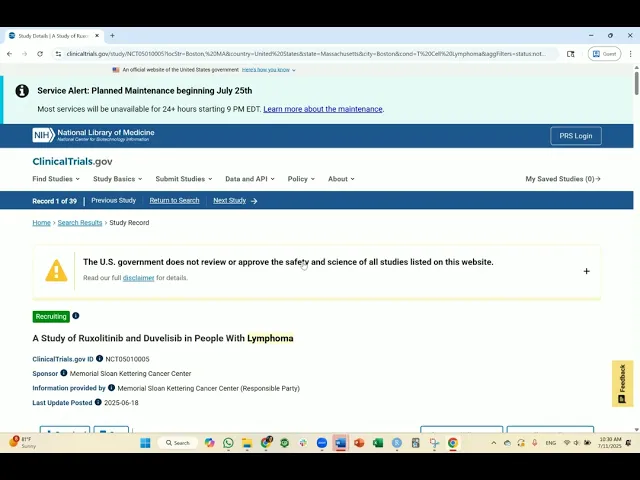
The best way to find clinical trials is at ClinicalTrials.gov. Here, you can search for clinicals based on your diagnosis and the location you prefer.
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
What information do I need in order to start applying for clinical trials?
In order to start applying for clinical trials, here are some general tasks to complete before beginning the search process:
1) Talk with your physician or care team about pursuing clinical trials and specific ones that might best fit your situation. They might also be able to provide additional resources to help you with the process.
2) Gather all of your medical records in one, organized place. This includes all laboratory tests, imaging, immunization records, medication lists, diagnoses, etc.
a. Tip: most hospital systems have an online portal where you can find all of your medical records and download them. It might also be helpful to write down all of the results in a spreadsheet or notebook to access the information more quickly.
3) Navigate to ClinicalTrials.gov to begin searching for clinical trials.
What keywords should I use when searching for a clinical trial?
You’ll want to start off using broader keywords related to T-cell lymphoma so that you can see the most results and find the clinical trial that is right for you. Some examples of keywords are:
T-cell Lymphoma
Peripheral T-cell Lymphoma
Angioimmunoblastic T-cell Lymphoma
Natural killer (NK)/T-cell Lymphoma
Large granular lymphocytic leukemia
Cutaneous T-cell Lymphoma
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
How do I know if I am eligible for a clinical trial?
I’ve found a clinical trial that I am eligible for. How do I sign up?
I do not have some of the information that the study requires in order to be eligible (i.e., laboratory tests, genetic information, etc.). What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
I meet most of the eligibility criteria for a clinical trial, but not all. Should I still contact the primary investigator?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
How do I know how long the clinical trial will take?
I’ve sent an email to the primary study contact and haven’t received a response. What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
How do I find clinical trials?
The best way to find clinical trials is at ClinicalTrials.gov. Here, you can search for clinicals based on your diagnosis and the location you prefer.
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
What information do I need in order to start applying for clinical trials?
In order to start applying for clinical trials, here are some general tasks to complete before beginning the search process:
1) Talk with your physician or care team about pursuing clinical trials and specific ones that might best fit your situation. They might also be able to provide additional resources to help you with the process.
2) Gather all of your medical records in one, organized place. This includes all laboratory tests, imaging, immunization records, medication lists, diagnoses, etc.
a. Tip: most hospital systems have an online portal where you can find all of your medical records and download them. It might also be helpful to write down all of the results in a spreadsheet or notebook to access the information more quickly.
3) Navigate to ClinicalTrials.gov to begin searching for clinical trials.
What keywords should I use when searching for a clinical trial?
You’ll want to start off using broader keywords related to T-cell lymphoma so that you can see the most results and find the clinical trial that is right for you. Some examples of keywords are:
T-cell Lymphoma
Peripheral T-cell Lymphoma
Angioimmunoblastic T-cell Lymphoma
Natural killer (NK)/T-cell Lymphoma
Large granular lymphocytic leukemia
Cutaneous T-cell Lymphoma
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
How do I know if I am eligible for a clinical trial?
I’ve found a clinical trial that I am eligible for. How do I sign up?
I do not have some of the information that the study requires in order to be eligible (i.e., laboratory tests, genetic information, etc.). What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
I meet most of the eligibility criteria for a clinical trial, but not all. Should I still contact the primary investigator?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
How do I know how long the clinical trial will take?
I’ve sent an email to the primary study contact and haven’t received a response. What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
How do I find clinical trials?
The best way to find clinical trials is at ClinicalTrials.gov. Here, you can search for clinicals based on your diagnosis and the location you prefer.
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
What information do I need in order to start applying for clinical trials?
In order to start applying for clinical trials, here are some general tasks to complete before beginning the search process:
1) Talk with your physician or care team about pursuing clinical trials and specific ones that might best fit your situation. They might also be able to provide additional resources to help you with the process.
2) Gather all of your medical records in one, organized place. This includes all laboratory tests, imaging, immunization records, medication lists, diagnoses, etc.
a. Tip: most hospital systems have an online portal where you can find all of your medical records and download them. It might also be helpful to write down all of the results in a spreadsheet or notebook to access the information more quickly.
3) Navigate to ClinicalTrials.gov to begin searching for clinical trials.
What keywords should I use when searching for a clinical trial?
You’ll want to start off using broader keywords related to T-cell lymphoma so that you can see the most results and find the clinical trial that is right for you. Some examples of keywords are:
T-cell Lymphoma
Peripheral T-cell Lymphoma
Angioimmunoblastic T-cell Lymphoma
Natural killer (NK)/T-cell Lymphoma
Large granular lymphocytic leukemia
Cutaneous T-cell Lymphoma
For more information, please watch this video that outlines the step-by-step process of finding clinical trials on ClinicalTrials.gov.
How do I know if I am eligible for a clinical trial?
I’ve found a clinical trial that I am eligible for. How do I sign up?
I do not have some of the information that the study requires in order to be eligible (i.e., laboratory tests, genetic information, etc.). What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
I meet most of the eligibility criteria for a clinical trial, but not all. Should I still contact the primary investigator?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
How do I know how long the clinical trial will take?
I’ve sent an email to the primary study contact and haven’t received a response. What should I do?
Under the “Participant Criteria” section of each clinical trial, you can see the list of eligibility criteria for the study.
Logistical Questions About Clinical Trials
How do I know the cost of participating in the clinical trial?
enter text
If the clinical trial takes place far away from where I live, can I receive financial assistance to travel to the trial?
You may be able to receive financial assistance for your commute to the trial center, as well as potential coverage of other costs like housing or childcare. You can ask the Study Contact of the clinical trial or check out some of these resources at the National Cancer Institute to see if you qualify for financial assistance:
Tracking Care Costs and Financial Assistance
Phone: 1-800-4-CANCER
Email: NCIinfo@nih.gov
You can also look at this non-profit that helps patients cover transportation costs for medical treatments.
I don’t speak English fluently. What resources do I have to find a clinical trial that can accommodate my language needs?
If you don’t speak English fluently, it is imperative that you are offered interpreter services. Even if you have a family member who is more fluent in English, they may not be able to accurately translate exactly what the researcher is asking from you or convey the severity of what is being said. You can ask the main research contact if they can provide interpretation services, and whether or not they can cover the cost. You can also ask your care team or insurance provider if interpretation services can be covered.
I’ve just been rejected from a clinical trial. What are my next steps?
Not being accepted into a clinical trial can feel disappointing and even hurtful. These are completely valid emotions that take time to heal from. Many times, participants are not chosen for reasons out of their control. For example, researchers might choose participants based on how well they fit the study criteria, and it’s possible that the study wasn’t the best fit for you. It’s also possible that the study received a large number of applicants, and couldn’t accept you even if you fit the study criteria well. The important thing to remember is that this is not the end, as there are always several new trials being published each week or month that are looking to recruit participants. Take time to acknowledge your emotions—process them independently, with loved ones, or with your care team. When you're ready, you can explore other clinical trials or follow your care team’s recommended next steps.
I cannot find any actively recruiting clinical trials that I am eligible for. What are my next steps?
There are always several clinical trials that are being published and are actively recruiting participants each month, so continue to check regularly. A tip is to set aside one hour each week to look at ClinicalTrials.gov and see if any new trials are published.
It might also be worthwhile to edit your search terms and look for trials that are located further away from you. Sometimes, researchers may cover your travel expenses so you can participate in a certain clinical trial.
What are additional resources available to me as I apply for clinical trials?
Always remember that your physician and care team are a great resource to use as you apply for clinical trials. Several hospitals also have dedicated staff or websites to help you navigate the clinical trial process as well. Here are some additional online resources:
Medical transportation financial assistance:
Help with finding clinical trials:
https://www.patientadvocate.org/
Basics of clinical trials:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
How do I know the cost of participating in the clinical trial?
enter text
If the clinical trial takes place far away from where I live, can I receive financial assistance to travel to the trial?
You may be able to receive financial assistance for your commute to the trial center, as well as potential coverage of other costs like housing or childcare. You can ask the Study Contact of the clinical trial or check out some of these resources at the National Cancer Institute to see if you qualify for financial assistance:
Tracking Care Costs and Financial Assistance
Phone: 1-800-4-CANCER
Email: NCIinfo@nih.gov
You can also look at this non-profit that helps patients cover transportation costs for medical treatments.
I don’t speak English fluently. What resources do I have to find a clinical trial that can accommodate my language needs?
If you don’t speak English fluently, it is imperative that you are offered interpreter services. Even if you have a family member who is more fluent in English, they may not be able to accurately translate exactly what the researcher is asking from you or convey the severity of what is being said. You can ask the main research contact if they can provide interpretation services, and whether or not they can cover the cost. You can also ask your care team or insurance provider if interpretation services can be covered.
I’ve just been rejected from a clinical trial. What are my next steps?
Not being accepted into a clinical trial can feel disappointing and even hurtful. These are completely valid emotions that take time to heal from. Many times, participants are not chosen for reasons out of their control. For example, researchers might choose participants based on how well they fit the study criteria, and it’s possible that the study wasn’t the best fit for you. It’s also possible that the study received a large number of applicants, and couldn’t accept you even if you fit the study criteria well. The important thing to remember is that this is not the end, as there are always several new trials being published each week or month that are looking to recruit participants. Take time to acknowledge your emotions—process them independently, with loved ones, or with your care team. When you're ready, you can explore other clinical trials or follow your care team’s recommended next steps.
I cannot find any actively recruiting clinical trials that I am eligible for. What are my next steps?
There are always several clinical trials that are being published and are actively recruiting participants each month, so continue to check regularly. A tip is to set aside one hour each week to look at ClinicalTrials.gov and see if any new trials are published.
It might also be worthwhile to edit your search terms and look for trials that are located further away from you. Sometimes, researchers may cover your travel expenses so you can participate in a certain clinical trial.
What are additional resources available to me as I apply for clinical trials?
Always remember that your physician and care team are a great resource to use as you apply for clinical trials. Several hospitals also have dedicated staff or websites to help you navigate the clinical trial process as well. Here are some additional online resources:
Medical transportation financial assistance:
Help with finding clinical trials:
https://www.patientadvocate.org/
Basics of clinical trials:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
How do I know the cost of participating in the clinical trial?
enter text
If the clinical trial takes place far away from where I live, can I receive financial assistance to travel to the trial?
You may be able to receive financial assistance for your commute to the trial center, as well as potential coverage of other costs like housing or childcare. You can ask the Study Contact of the clinical trial or check out some of these resources at the National Cancer Institute to see if you qualify for financial assistance:
Tracking Care Costs and Financial Assistance
Phone: 1-800-4-CANCER
Email: NCIinfo@nih.gov
You can also look at this non-profit that helps patients cover transportation costs for medical treatments.
I don’t speak English fluently. What resources do I have to find a clinical trial that can accommodate my language needs?
If you don’t speak English fluently, it is imperative that you are offered interpreter services. Even if you have a family member who is more fluent in English, they may not be able to accurately translate exactly what the researcher is asking from you or convey the severity of what is being said. You can ask the main research contact if they can provide interpretation services, and whether or not they can cover the cost. You can also ask your care team or insurance provider if interpretation services can be covered.
I’ve just been rejected from a clinical trial. What are my next steps?
Not being accepted into a clinical trial can feel disappointing and even hurtful. These are completely valid emotions that take time to heal from. Many times, participants are not chosen for reasons out of their control. For example, researchers might choose participants based on how well they fit the study criteria, and it’s possible that the study wasn’t the best fit for you. It’s also possible that the study received a large number of applicants, and couldn’t accept you even if you fit the study criteria well. The important thing to remember is that this is not the end, as there are always several new trials being published each week or month that are looking to recruit participants. Take time to acknowledge your emotions—process them independently, with loved ones, or with your care team. When you're ready, you can explore other clinical trials or follow your care team’s recommended next steps.
I cannot find any actively recruiting clinical trials that I am eligible for. What are my next steps?
There are always several clinical trials that are being published and are actively recruiting participants each month, so continue to check regularly. A tip is to set aside one hour each week to look at ClinicalTrials.gov and see if any new trials are published.
It might also be worthwhile to edit your search terms and look for trials that are located further away from you. Sometimes, researchers may cover your travel expenses so you can participate in a certain clinical trial.
What are additional resources available to me as I apply for clinical trials?
Always remember that your physician and care team are a great resource to use as you apply for clinical trials. Several hospitals also have dedicated staff or websites to help you navigate the clinical trial process as well. Here are some additional online resources:
Medical transportation financial assistance:
Help with finding clinical trials:
https://www.patientadvocate.org/
Basics of clinical trials:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
How do I know the cost of participating in the clinical trial?
enter text
If the clinical trial takes place far away from where I live, can I receive financial assistance to travel to the trial?
You may be able to receive financial assistance for your commute to the trial center, as well as potential coverage of other costs like housing or childcare. You can ask the Study Contact of the clinical trial or check out some of these resources at the National Cancer Institute to see if you qualify for financial assistance:
Tracking Care Costs and Financial Assistance
Phone: 1-800-4-CANCER
Email: NCIinfo@nih.gov
You can also look at this non-profit that helps patients cover transportation costs for medical treatments.
I don’t speak English fluently. What resources do I have to find a clinical trial that can accommodate my language needs?
If you don’t speak English fluently, it is imperative that you are offered interpreter services. Even if you have a family member who is more fluent in English, they may not be able to accurately translate exactly what the researcher is asking from you or convey the severity of what is being said. You can ask the main research contact if they can provide interpretation services, and whether or not they can cover the cost. You can also ask your care team or insurance provider if interpretation services can be covered.
I’ve just been rejected from a clinical trial. What are my next steps?
Not being accepted into a clinical trial can feel disappointing and even hurtful. These are completely valid emotions that take time to heal from. Many times, participants are not chosen for reasons out of their control. For example, researchers might choose participants based on how well they fit the study criteria, and it’s possible that the study wasn’t the best fit for you. It’s also possible that the study received a large number of applicants, and couldn’t accept you even if you fit the study criteria well. The important thing to remember is that this is not the end, as there are always several new trials being published each week or month that are looking to recruit participants. Take time to acknowledge your emotions—process them independently, with loved ones, or with your care team. When you're ready, you can explore other clinical trials or follow your care team’s recommended next steps.
I cannot find any actively recruiting clinical trials that I am eligible for. What are my next steps?
There are always several clinical trials that are being published and are actively recruiting participants each month, so continue to check regularly. A tip is to set aside one hour each week to look at ClinicalTrials.gov and see if any new trials are published.
It might also be worthwhile to edit your search terms and look for trials that are located further away from you. Sometimes, researchers may cover your travel expenses so you can participate in a certain clinical trial.
What are additional resources available to me as I apply for clinical trials?
Always remember that your physician and care team are a great resource to use as you apply for clinical trials. Several hospitals also have dedicated staff or websites to help you navigate the clinical trial process as well. Here are some additional online resources:
Medical transportation financial assistance:
Help with finding clinical trials:
https://www.patientadvocate.org/
Basics of clinical trials:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
How do I know the cost of participating in the clinical trial?
enter text
If the clinical trial takes place far away from where I live, can I receive financial assistance to travel to the trial?
You may be able to receive financial assistance for your commute to the trial center, as well as potential coverage of other costs like housing or childcare. You can ask the Study Contact of the clinical trial or check out some of these resources at the National Cancer Institute to see if you qualify for financial assistance:
Tracking Care Costs and Financial Assistance
Phone: 1-800-4-CANCER
Email: NCIinfo@nih.gov
You can also look at this non-profit that helps patients cover transportation costs for medical treatments.
I don’t speak English fluently. What resources do I have to find a clinical trial that can accommodate my language needs?
If you don’t speak English fluently, it is imperative that you are offered interpreter services. Even if you have a family member who is more fluent in English, they may not be able to accurately translate exactly what the researcher is asking from you or convey the severity of what is being said. You can ask the main research contact if they can provide interpretation services, and whether or not they can cover the cost. You can also ask your care team or insurance provider if interpretation services can be covered.
I’ve just been rejected from a clinical trial. What are my next steps?
Not being accepted into a clinical trial can feel disappointing and even hurtful. These are completely valid emotions that take time to heal from. Many times, participants are not chosen for reasons out of their control. For example, researchers might choose participants based on how well they fit the study criteria, and it’s possible that the study wasn’t the best fit for you. It’s also possible that the study received a large number of applicants, and couldn’t accept you even if you fit the study criteria well. The important thing to remember is that this is not the end, as there are always several new trials being published each week or month that are looking to recruit participants. Take time to acknowledge your emotions—process them independently, with loved ones, or with your care team. When you're ready, you can explore other clinical trials or follow your care team’s recommended next steps.
I cannot find any actively recruiting clinical trials that I am eligible for. What are my next steps?
There are always several clinical trials that are being published and are actively recruiting participants each month, so continue to check regularly. A tip is to set aside one hour each week to look at ClinicalTrials.gov and see if any new trials are published.
It might also be worthwhile to edit your search terms and look for trials that are located further away from you. Sometimes, researchers may cover your travel expenses so you can participate in a certain clinical trial.
What are additional resources available to me as I apply for clinical trials?
Always remember that your physician and care team are a great resource to use as you apply for clinical trials. Several hospitals also have dedicated staff or websites to help you navigate the clinical trial process as well. Here are some additional online resources:
Medical transportation financial assistance:
Help with finding clinical trials:
https://www.patientadvocate.org/
Basics of clinical trials:
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
Find PTCL Care Centers Near You
Take me there
